Explain the Difference Between Emission and Absorption Spectra
These emitted photons give emission spectrum. Absorption involves the absorbing of energy by the electrons.
What Is The Difference Between Emission Spectra And Absorption Spectra Hubpages
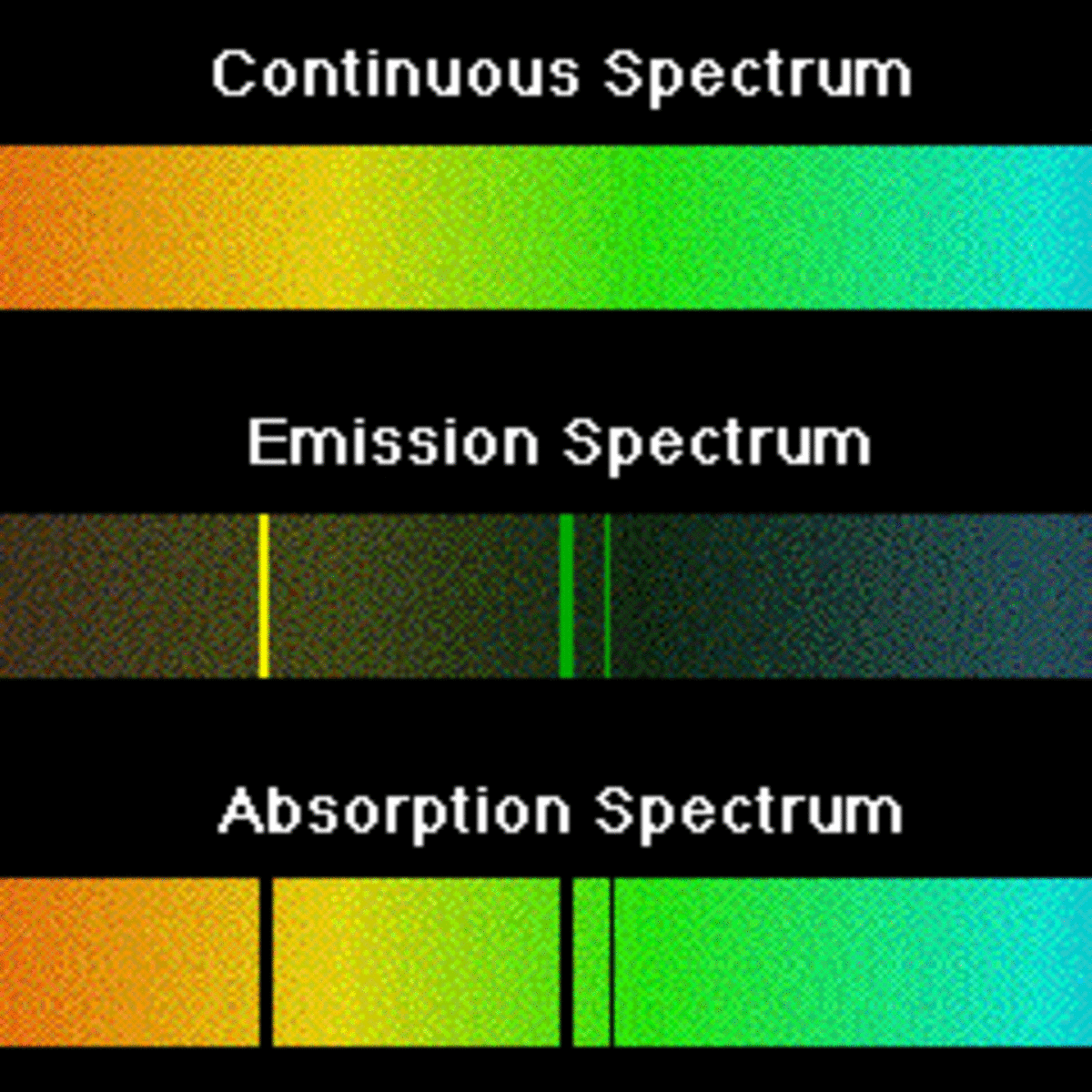
The key difference between the spectrum of emission and absorption is that the spectrum of emission has different coloured lines in the spectrum while the spectrum of absorption has dark-coloured lines in the spectrum.
. Continuum Absorption Emission Spectra. Δλ 1 nm. 55 Explain how emission line spectra and absorption line spectra are formed.
4 rows The major difference between the emission spectrum and the absorption spectrum is that. We may view a continuum spectrum as an emission spectrum in which the lines overlap with each other and can no longer be distinguished as individual emission lines. When an electron from a lower level wants to rise to a higher level it must absorb passing waves of photons to do so and we see this with the.
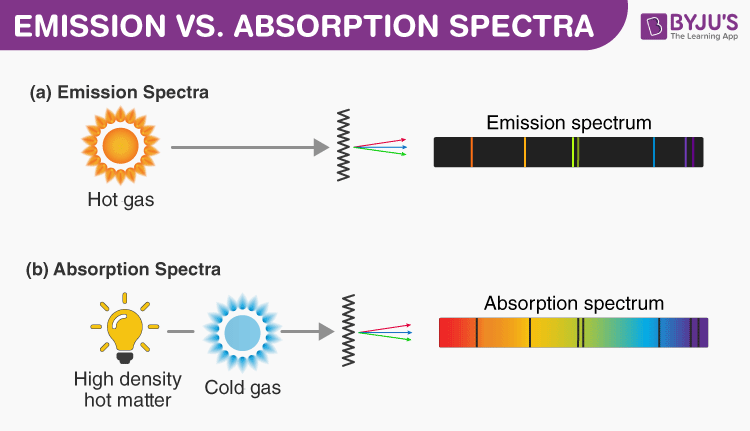
Photon absorption provides the energy for electrons to climb the set of energy levels for that element. The difference between absorption and emission spectra are that absorption lines are where light has been absorbed by the atom thus you see a dip in the spectrum whereas emission spectra have spikes in the spectra due to atoms releasing photons at those wavelengths. These frequencies are related to energy difference between excited state and lowe.
The basic difference between emission and absorption spectrum is as the name suggests emission and absorption of light. 2Both use a light source and a spectrophotometer. 4Emission spectra emit.
In this blog post the differences between molecular absorption excitation and emission spectra are explained. The excitation spectrum closely resembles an absorption spectrum since the emission intensity is usually proportional to the absorbance of the molecule. Mark me as brainliest.
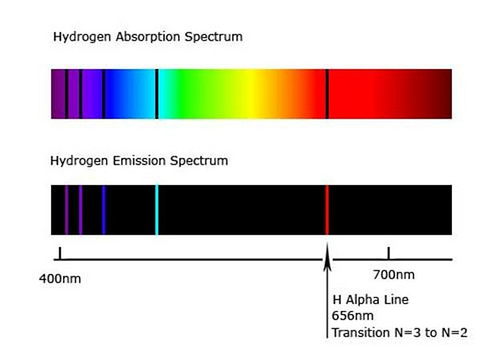
Answer 1 of 9. A given atom will absorb and emit the SAME frequencies of electromagnetic E-M radiation. An absorption spectrum occurs when light passes through a cold dilute gas and atoms in the gas absorb at characteristic frequencies.
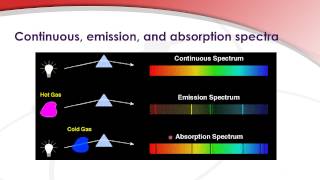
Join Login Class 11 Chemistry Structure of Atom Evidence for Quantized Electronic Energy Levels Explain the differences bet. In simple terms absorption spectra records the wavelengths absorbed by the material whereas emission spectra records wavelengths emitted by materials which have been stimulated by energy before. If you were to.
So putting electrons into higher energy states within an atom. Absorption spectrum of anthracene in cyclohexane measured using the FS5 Spectrofluorometer. 10 rows Emission can happen in the form of light and rays such as gamma and radio.
In an excitation spectrum the emission is measured at one wavelength while the excitation wavelengths are scanned. The spectrum is a. 1Emission and absorption spectra can both be used in determining the composition of matter.
When atoms or molecules are excited and in higher energy state they emit photons to get deexcited. Difference between absorption and emission spectra atomic physicsOur MantraInformation is OpportunityKnowledge is PowerBe Informed - Be Powerful. Explain Kirchhoffs three laws and the difference between continuous emission and absorption spectra.
Difference Between and Emission Definition. Since the re-emitted light is unlikely to be emitted in the same. When electrons emit energy they move down towards a lower energy level.
Explain the differences between emission and absorption spectra. Click hereto get an answer to your question Explain the differences between emission and absorption spectra. When electrons absorb energy they leap to higher energy levels.
But theres more to it. Emission and absorption line spectra are formed from photons giving energy to excite the electrons in an atom. When electrons absorb energy they move up towards a higher energy level.
In the tabular column further differences between absorption and emission spectrum are given below. What is the cause of spectral lines Question. This type of spectrum is called an emission spectrum.
3Emission spectra measure the wavelength of the emitted light after the atoms are excited with heat while absorption. Consequently an emission spectra is a series of specific single color lines against a black background for each of the emitted frequencies. Explain Kirchhoffs three laws and the difference between continuous emission and absorption spectra.
It consists of light containing several frequencies. A gas of hydrogen atoms will produce an absorption line spectrum if it is between you your telescopespectrograph and a continuum light source and an emission line spectrum if viewed from a different angle. The major distinction between emission and absorption spectra is that an emission spectrum has various colored lines whereas an absorption spectrum contains dark-colored lines.
One of the major differences between the absorption spectrum and the emission spectrum is that the absorption spectrum has dark lines and the emission spectrum has different coloured lines. Some parts of the light spectrum can be seen by animals but not by humans. When electrons return to their original energy levels this is called emission.
For example certain insects can see UV light while we cannot. Emission refers to the release of energy by the electrons.

6 3 Atomic Line Spectra And Niels Bohr Chemistry Libretexts

What Is The Difference Between Emission And Absorption Spectrum In Tabular Form Plzzzz Chemistry Structure Of Atom 13435451 Meritnation Com

Explain The Difference Between Emission And Absorpation Spectra

Difference Between Emission Spectra And Absorption Spectra Chemistry 1 Brainly In

Difference Between Flame Emission Spectroscopy And Atomic Absorption Spectroscopy Compare The Difference Between Similar Terms
Difference Between Absorption And Emission Spectra Definition Characteristics Comparison
Difference Between Continuous Spectrum And Line Spectrum Pediaa Com
Difference Between Emission And Absorption Spectra Comparison Chart

Spectroscopy Why Absorption Spectum Is Not Identical To Emission Spectrum Physics Stack Exchange

Explain The Difference Between Emission And Absorpation Spectra

Difference Between Emission And Absorption Spectra Comparison Chart

Diffrence Between Emission And Absorption Spectrum Brainly In

2 3 2 Distinguish Between A Continuous Spectrum And A Line Spectrum Youtube

What Is The Difference Between Absorption And Emission Spectra Atomic Physics Youtube
Absorption Spectrum Emission Spectrum Lines Article Khan Academy

Emission Spectrum Vs Absorption Spectrum Astronomy Lessons Emissions Spectrum

Difference Between Excitation And Absorption Compare The Difference Between Similar Terms
Difference Between Emission And Absorption Spectra Difference Between
Difference Between Absorption And Emission Spectra Definition Characteristics Comparison
Comments
Post a Comment